Book Your Dermal Filler Session with Dr. Laura Geige Today
Age Requirements and Guidelines
The age requirements for lip fillers vary depending on the country, state, or region you are in, as well as the specific procedure being performed.
In general, most healthcare providers and dermatologists require patients to be at least 18 years old to undergo lip filler procedures, as this is considered the legal age of consent in most countries.
However, some clinics or spas may offer lip fillers to younger patients, typically those in their early teens, under certain conditions and with parental consent.
The American Society for Dermatologic Surgery (ASDS) recommends that patients be at least 21 years old for injectable fillers, including lip fillers, due to the potential risks associated with these procedures.
The FDA also has specific guidelines for the use of dermal fillers in patients under the age of 22, stating that these products are not approved for use in minors and should only be used by individuals who are at least 18 years old.
It’s worth noting that lip filler requirements can vary depending on the type of filler being used. For example, hyaluronic acid fillers, which are the most common type of lip filler, can usually be used in patients as young as 16 or 17 with parental consent.
Permanent fillers, such as silicone or PMMA, typically require patients to be at least 21 years old due to the potential risks and long-term effects associated with these substances.
Regardless of age, patients under the age of 18 should carefully consider the following before undergoing a lip filler procedure:
-Potential risks and complications, such as infection, scarring, or temporary numbness;
-The permanence of the results, as some fillers can last for many years;
-The potential impact on their self-esteem and body image;
-The need for repeated treatments to maintain the desired results.
Ultimately, patients should consult with a qualified healthcare professional or dermatologist to discuss their individual needs and determine if lip fillers are right for them, regardless of age.
It’s also essential to choose a reputable and licensed clinic or spa that follows proper safety protocols and guidelines to minimize the risk of complications.
Lip Filler Age Requirements vary depending on the country, state, and even region. In general, most healthcare professionals and medical spas require patients to be at least 18 years old to undergo lip filler procedures.
The American Society for Dermatologic Surgery (ASDS) recommends that patients under the age of 25 should not receive dermal fillers, including lip fillers, due to skin laxity and the possibility of _permanent scarring_.
For those 25-34 years old, a consultation with a board-certified dermatologist or plastic surgeon is recommended to determine if lip fillers are suitable for their individual skin concerns and needs.
Patients between 35-44 years old can typically receive lip filler treatments, but may require more maintenance sessions due to loss of collagen and _natural aging processes_.
For individuals over 45 years old, the risks associated with dermal fillers increase, and they should carefully weigh the potential benefits against the possible complications.
Guidelines for Lip Filler Procedures include:
- Morning sessions are preferred: Lip filler procedures should be performed in the morning to minimize swelling and allow for optimal results.
- Sedation may not be necessary: While some patients may require sedation, others can undergo lip filler treatments without it. Your healthcare professional will advise you on the best course of action.
- Avoiding certain medications: Certain medications, such as _blood thinners_, can increase the risk of complications during and after the procedure. Inform your healthcare professional about any medications you’re taking.
- Follow post-procedure instructions: To ensure optimal results and minimize side effects, follow your healthcare professional’s instructions regarding rest, nutrition, and avoiding strenuous activities.
Risks and Complications associated with lip filler procedures include:
- Infection: As with any invasive medical procedure, there is a risk of infection with lip fillers.
- Nerve damage: The nerves in the face can be irritated during lip filler procedures, leading to numbness or permanent nerve damage.
- Scarring: While rare, scarring can occur with lip filler procedures.
Long-term Effects of lip fillers include:
- Loss of sensation: The numbness or loss of sensation in the lips can be permanent and may require additional treatments.
- Swollen lips: Lip swelling is a common side effect that can last for several days to weeks after the procedure.
- Skin irregularities: Irregularities in skin texture or scarring can occur, especially if the procedure is not performed by an experienced healthcare professional.
The minimum age requirement for lip filler injections varies significantly across different countries and jurisdictions, with some allowing minors to undergo the procedure while others prohibit it altogether.
In the United States, for instance, the American Society of Plastic Surgeons (ASPS) recommends that individuals be at least 18 years old to receive lip fillers. This is because the ASPS emphasizes the importance of informed consent, and minors are typically not considered capable of providing this consent.
However, some states in the US have more lenient regulations. In California, for example, minors can undergo lip filler procedures as long as they meet certain criteria, such as having a parent or guardian present to provide written consent.
In contrast, several European countries have stricter age requirements. In the UK, for instance, the British Association of Aesthetic Plastic Surgeons (BAAPS) advises that individuals be at least 18 years old to receive lip fillers, with some exceptions for individuals with specific medical conditions or disabilities.
Canada has a similar approach, with Health Canada regulating the use of fillers and requiring that minors undergo special authorization procedures before undergoing the procedure. In general, this means that parents or guardians must provide written consent and attend the procedure with their minor child.
Australia also has its own set of regulations surrounding lip filler use among minors. The Australian Medical Association (AMA) advises that individuals be at least 18 years old to receive fillers, with some exceptions for medical or cosmetic purposes.
In many Asian countries, such as Japan and South Korea, there are no specific age restrictions on lip filler use among minors. However, the ASPS cautions that these countries may have different regulations and guidelines in place, which are not always clearly communicated to patients.
It is essential for individuals considering lip fillers, regardless of age, to consult with a qualified healthcare professional or licensed aesthetic practitioner to discuss their specific options and any relevant health concerns.
Reserve a Dermal Filler Appointment with Dr. Laura Geige Now
Additionally, some states have laws regulating the use of fillers among minors. For example, in New York, minors under the age of 18 require written consent from a parent or guardian to undergo lip filler procedures.
In terms of guidelines, the American Society for Dermatologic Surgery (ASDS) suggests that individuals be at least 21 years old to receive fillers, with some exceptions for medical purposes. The ASDS emphasizes the importance of proper patient education and aftercare instructions.
The minimum age requirement for non-injectable lip filler treatments is 18 years old, as recommended by the American Society for Dermatologic Surgery (ASDS).
This guideline takes into account the developing skin and bone structure of younger individuals, which may not be suitable for certain types of treatments.
On the other hand, injectable lip fillers, such as
The reason for this age requirement is primarily due to the maturity of the skin’s collagen production and the brain’s ability to make informed decisions about medical treatments.
In terms of injectable lip fillers, it is recommended that patients be at least 22-25 years old for optimal results. This allows enough time for the body to adjust to the treatment and minimizes the risk of complications such as granuloma formation.
Additionally, some studies have shown that younger individuals may experience a higher rate of necrosis or tumor formation after receiving injectable lip fillers due to their immature skin structure.
It’s also worth noting that certain medical conditions, such as a history of bleeding disorders or autoimmune diseases, may affect an individual’s suitability for lip filler treatments and require additional evaluation by a qualified healthcare professional.
In summary, while 18-year-olds can undergo non-injectable lip filler treatments, injectable treatments are typically reserved for patients aged 21 years and above due to the maturity of their skin structure and the brain’s ability to make informed decisions about medical treatments.
The American Society for Dermatologic Surgery (ASDS) recommends that patients under 25 years old should exercise caution when considering lip fillers, as they are more prone to experiencing adverse reactions.
According to a study published in the Journal of Clinical and Aesthetic Dermatology, the risk of adverse reactions to lip fillers increases significantly with age. The researchers found that patients under 25 were more likely to experience complications, such as swelling, bruising, and scarring, compared to those over 25.
The study also noted that young adults are more susceptible to filler migration, which occurs when the fillers move from the original injection site to another area of the face. This can lead to an uneven appearance and may require additional procedures to correct.
Another concern is the potential for lip fillers to cause permanent damage in younger patients. The American Society for Dermatologic Surgery notes that young adults’ skin is more delicate and has a greater capacity to stretch, which can lead to lumps or irregularities under the skin.
Additionally, some lip fillers are not approved for use on facial areas near the mouth or nose in individuals under 25. These products may contain agents that are not suitable for young skin, increasing the risk of adverse reactions.
The FDA recommends that patients over 18 years old receive lip fillers from a qualified healthcare professional with experience in administering these treatments. However, this guideline does not specifically address the age restrictions imposed by some product manufacturers.
Some popular lip filler brands have their own set of guidelines for patient age. For example, Juvederm recommends that patients under 22 years old should not use its products due to concerns about potential complications.
Other countries and regions may have different guidelines or regulations regarding the use of lip fillers in younger patients. It is essential for individuals under 25 to carefully consider their options and consult with a qualified healthcare professional before undergoing any cosmetic procedures.
To minimize risks, it is crucial for young adults to choose a board-certified dermatologist, plastic surgeon, or other licensed medical professional who has experience with lip fillers. A thorough evaluation and discussion of the potential benefits and risks should occur prior to treatment.
Age requirements for lip fillers vary depending on the type of filler used, the country, and the healthcare provider.
In general, most medical professionals consider patients to be at least 18 years old to administer Lip Fillers that contain hyaluronic acid, such as Juvederm or Restylane.
This is because hyaluronic acid fillers are considered to be relatively safe and effective for most adults, but may not be suitable for minors due to the risk of allergic reactions or other complications.
However, some countries have different age requirements. For example, in the United Kingdom, patients under the age of 21 must obtain written permission from a parent or guardian before undergoing lip augmentation procedures using Lip Fillers.
In addition to the minimum age requirement, there may be other guidelines that healthcare providers follow when administering lip fillers.
These include:
Ensuring that patients are in good overall health and have realistic expectations about the procedure;
Evaluating patients’ medical history and any previous allergic reactions or sensitivities they may have to certain ingredients;
Determining the best type of filler for each individual patient, taking into account their skin type, lip shape, and desired results;
Providing thorough instructions on post-procedure care and aftercare
Miscellaneous procedures, such as Lip Blushing or permanent lip fillers, may have different age requirements. These procedures are often performed for aesthetic purposes only.
Some healthcare providers may consider patients to be mature enough to undergo these procedures at a younger age, typically around the mid-to-late teens, but this ultimately depends on individual circumstances and parental consent.
It’s also worth noting that some Lip Fillers contain other ingredients, such as collagen or calcium hydroxylapatite, which may have different age requirements due to varying levels of safety and efficacy data.
Cosmetic lip fillers are generally considered to be a cosmetic treatment, rather than a medical one, so there may not be the same level of oversight or regulation as with certain medical procedures.
This can make it more difficult for patients to determine whether they meet the age requirements and guidelines for their specific procedure.
In any case, patients should always consult with a qualified healthcare provider before undergoing lip augmentation procedures to discuss their individual needs and circumstances.
Health Considerations and Consent
The decision to undergo any medical procedure, including lip filler injections, should be carefully considered and informed by a thorough understanding of the potential health considerations and consent requirements.
A person seeking to have lip fillers must first ensure they meet certain pre-procedure requirements to minimize risks and optimize outcomes.
One of the most critical health considerations is age. While there is no specific upper age limit for lip filler procedures, individuals over 65 years old may be more susceptible to complications due to natural aging processes and potential underlying health conditions.
For example, older adults may experience decreased skin elasticity, increased inflammation, and a higher risk of scarring or infection due to weaker immune systems. In such cases, it is essential for patients to discuss their individual situation with their healthcare provider or dermatologist before making a decision.
A thorough medical evaluation, including a review of medical history, medications, and any pre-existing conditions, should be conducted by a qualified healthcare professional to ensure the individual is healthy enough for the procedure.
Additionally, patients under 18 years old will typically need written consent from their parents or guardians prior to undergoing lip filler procedures. This ensures that informed decision-making takes place and respects the minor’s rights and well-being.
A detailed discussion about potential risks and benefits should also occur with a healthcare provider to ensure that patients fully comprehend what is involved in the procedure, including the likelihood of temporary or permanent results, possible side effects, and necessary follow-up care.
Patients must be aware that certain medical conditions, such as autoimmune disorders (e.g., lupus, rheumatoid arthritis), bleeding disorders (e.g., hemophilia), or neurological issues may increase the risk associated with lip filler procedures. In these cases, alternative treatment options or modifications to the original plan should be explored under the guidance of a qualified healthcare professional.
Furthermore, individuals taking certain medications that affect blood clotting, platelet aggregation, or anti-inflammatory responses should also discuss potential interactions and risks related to the procedure with their doctor beforehand.
Certain pre-procedure requirements may also apply to patients who have recently smoked or have a history of smoking-related health issues. In some cases, smokers may be at higher risk for certain complications following lip filler procedures due to compromised blood flow and reduced oxygen delivery to tissues.
Finally, the use of hyaluronic acid fillers carries some inherent risks, such as allergic reactions, infection, scarring, or lump formation, which can occur in a small percentage of individuals. In such instances, proper aftercare and follow-up with a healthcare provider are vital for ensuring optimal outcomes and minimizing long-term complications.
Lip fillers are a popular cosmetic treatment used to enhance the appearance of the lips by injecting hyaluronic acid or other substances into the lip tissues.
When it comes to health considerations and consent, it’s essential to discuss the potential risks associated with lip filler injections, especially in minors.
The American Society for Dermatologic Surgery (ASDS) states that individuals under the age of 18 should not receive dermal fillers, including lip fillers, without parental or guardian consent.
This is because minors may not fully understand the risks and potential complications associated with the treatment, nor can they provide informed consent.
Some potential risks associated with lip filler injections include infection, allergic reactions, scarring, and unevenness or asymmetry of the lips.
Furthermore, certain health conditions, such as cold sores, eczema, or rosacea, may increase the risk of complications from lip filler injections.
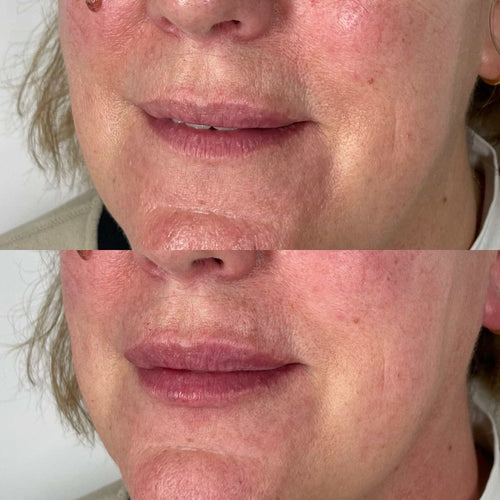
Additionally, individuals taking medications such as blood thinners, anticoagulants, or aspirin should not receive lip fillers, as these medications can increase the risk of bleeding or bruising at the injection site.
Healthcare providers must obtain informed consent from individuals before performing any cosmetic treatment, including lip filler injections.
Informed consent involves discussing the potential risks and benefits of the treatment, as well as alternative options, with the individual.
The FDA requires that all healthcare providers using dermal fillers, including lip fillers, follow strict guidelines to ensure patient safety.
These guidelines include proper training, licensure, and registration requirements for healthcare providers, as well as detailed instructions for preparing and performing the procedure.
Minors who wish to undergo lip filler injections should be accompanied by a parent or guardian, who must provide written consent on their behalf.
The parent or guardian must understand the potential risks and benefits of the treatment, as well as the responsibilities of the healthcare provider in ensuring the minor’s safety.
Healthcare providers may require minors to undergo additional screening, such as a physical examination, before performing lip filler injections.
This is to assess any underlying health conditions that may increase the risk of complications from the treatment.
In some cases, healthcare providers may recommend alternative treatments or modifications to the original plan to accommodate a minor’s individual needs and health status.
The decision to undergo any cosmetic procedure, including lip filler treatment, should not be taken lightly and requires careful consideration of both physical and mental health factors.
According to the British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS), patients must be in good physical and mental health before undergoing lip filler treatment. This includes having realistic expectations about the outcome of the treatment and being willing to follow post-operative instructions to ensure a smooth recovery.
Some of the key health considerations that must be taken into account include:
- A history of bleeding disorders or taking anticoagulant medications
- a history of allergies to the materials used in the treatment, such as collagen or hyaluronic acid
- active skin infections or wounds
- autoimmune disorders, such as rheumatoid arthritis or lupus
- previous scarring or keloid formation at the injection site
- a history of certain medical conditions, such as diabetes or hypertension
Mental health considerations are also important, as patients should be in a stable emotional state and free from excessive anxiety or stress before undergoing lip filler treatment.
A patient’s mental health can be affected by various factors, including:
- a history of mental health conditions, such as depression or anxiety
- current substance abuse or addiction issues
- recent traumatic events or significant life changes
The BAPRAS recommends that patients should be at least 18 years old to undergo lip filler treatment. This is because the lips are still developing at this age, and the risk of complications increases significantly after the age of 25.
However, it’s worth noting that some younger patients may be eligible for lip filler treatment if they have a thorough medical assessment and can demonstrate their ability to give informed consent.
Informed consent is a critical aspect of any cosmetic procedure, including lip filler treatment. Patients must fully understand the risks, benefits, and alternatives to the treatment before making an informed decision.
During the consultation, patients should be made aware of:
- The potential risks and complications associated with the treatment
- The expected outcome of the treatment and any variations that may occur
- The cost and payment options for the treatment
- Any necessary follow-up appointments or care after the procedure
Patients must also be aware of their right to refuse treatment at any time, without penalty or loss of benefit.
The decision to undergo lip filler procedures, such as hyaluronic acid fillers, is a personal one that requires careful consideration and informed consent from all parties involved.
Health considerations are paramount when it comes to any medical procedure, including lip fillers.
- Age: While there is no specific minimum age limit for undergoing lip filler procedures, patients under the age of 18 must obtain parental or guardian consent. Additionally, NICE recommends that patients receive counseling about the risks and benefits of the procedure before giving informed consent.
- Health status: Patients with certain medical conditions, such as diabetes, autoimmune disorders, or bleeding disorders, may be at increased risk for complications during and after the procedure.
- Pregnancy and breastfeeding: As with any invasive procedure, patients who are pregnant or breastfeeding should avoid lip filler treatments until their health provider advises it is safe to do so.
- Medications: Certain medications, such as blood thinners, can increase the risk of bleeding complications during and after the procedure.
- Allergies: Patients with known allergies to the materials used in the fillers or other substances may be at increased risk for an allergic reaction.
- Surgical history: Patients with a history of bleeding disorders or those who have had previous lip surgeries may be at increased risk for complications during and after the procedure.
Furthermore, patients should also consider their overall health and any underlying medical conditions that may affect the outcome of the procedure or increase the risk of complications.
The risks associated with lip filler procedures can include:
- Bleeding or bruising at the injection site
- Swelling, redness, or inflammation at the injection site
- Infection at the injection site
- Asymmetrical results
- Permanent scarring
In addition to these risks, NICE recommends that patients be informed about the potential long-term effects of lip fillers, such as migration of the filler material, deformation of the lips, or changes in facial expressions.
Informed consent is a crucial aspect of any medical procedure, including lip fillers. Patients must be fully aware of the risks and benefits associated with the treatment before making an informed decision about undergoing the procedure.
This includes discussing their health status, medication list, and any underlying medical conditions that may affect the outcome of the procedure or increase the risk of complications.
The use of _lip fillers_ has become increasingly popular in recent years, with many individuals seeking to enhance the appearance of their lips for cosmetic reasons.
However, as with any medical procedure, there are several health considerations and consent issues that need to be addressed.
To determine if a person is eligible for lip filler treatment, healthcare professionals must consider their overall _health status_ and _medical history_.
Potential _contraindications_ for lip filler treatment include:
- _Immunocompromised states_ such as HIV/AIDS or undergoing chemotherapy, which can increase the risk of infection or allergic reactions.
- _Hypersensitivity to the ingredients_ used in the lip filler, such as _calcium hydroxylapatite_ or _polylactic acid_.
- _Neuromuscular disorders_ such as _myasthenia gravis_, which can affect muscle function and response to treatment.
- _Pregnancy or breastfeeding_, as the effects of lip fillers on fetal development are not yet fully understood.
- _Current use of blood thinners or medications that affect platelet function_, which can increase the risk of bleeding complications.
Additionally, healthcare professionals must also consider a person’s _psychological_ and _emotional_ well-being before authorizing lip filler treatment.
Consent is a critical aspect of any medical procedure, including lip filler treatment.
_Informed consent_ requires that the individual understands the purpose, risks, benefits, and alternatives of the treatment, as well as any potential _side effects_ or complications.
The consent process should also take into account a person’s ability to make decisions about their own care, free from coercion or undue influence.
_Minor consent_ is generally not applicable for cosmetic procedures like lip filler treatment, and minors should be accompanied by a parent or guardian to ensure that they fully understand the implications of treatment.
In some jurisdictions, minors may require special permits or approvals from a _guardian ad litem_ or other authorized representative before undergoing lip filler treatment.
Healthcare professionals have a responsibility to obtain and document informed consent from individuals seeking lip filler treatment, ensuring that they are fully aware of the risks and benefits involved.
This includes providing clear information about:
- The _nature and purpose_ of the treatment
- _Risks and potential complications_ associated with lip filler treatment, such as swelling, bruising, or scarring.
- _Potential long-term effects_ of the treatment, although these are generally rare and temporary.
- _Alternatives to treatment_, such as lifestyle changes or other cosmetic procedures.
- _Post-treatment care and follow-up_, including any necessary _aftercare instructions_ or _follow-up appointments_.
By obtaining informed consent, healthcare professionals can ensure that individuals seeking lip filler treatment are fully aware of the risks and benefits involved, allowing them to make an educated decision about their own care.
Regulatory Oversight and Education
The regulation of cosmetic procedures, including lip fillers, varies by country and jurisdiction. In many places, regulatory oversight and education play a crucial role in ensuring the safe and effective use of these treatments.
In the United States, for example, the regulation of cosmetic procedures is primarily handled at the state level. Each state has its own board of medical examiners or licensing authorities that oversee the practice of medicine, including plastic surgery and cosmetic dermatology.
These boards typically require doctors to complete a certain number of hours of training in cosmetic procedures, including lip fillers, as part of their initial licensure and ongoing continuing education requirements.
Additionally, many states have specific laws and regulations governing the use of lip fillers, including requirements for labeling, marketing, and patient consent forms.
For non-medical practitioners, such as estheticians or dermatologists, who perform cosmetic procedures, regulatory oversight may involve different levels of education and training. For example, many states require estheticians to complete a certain number of hours of training in cosmetic procedures before they can perform them on clients.
Accreditation is also an important aspect of regulatory oversight in the cosmetic industry. Accrediting organizations, such as the American Society for Laser Medicine and Surgery (ASLMS) or the American Association for Cosmetic Surgery (AACS), provide standards for training and education programs, as well as quality control measures to ensure that practitioners are using lip fillers safely and effectively.
Some of the key regulations governing lip filler use include:
- The Federal Food, Drug, and Cosmetic Act requires all cosmetic products, including lip fillers, to be approved for safety and efficacy by the FDA before they can be marketed or sold in the United States.
- The American Society of Plastic Surgeons (ASPS) recommends that patients seeking lip fillers undergo a thorough medical evaluation, including a physical exam and discussion of potential risks and complications.
In terms of education, practitioners who perform lip filler procedures typically require a certain level of training in areas such as:
- Anatomy and physiology
- Cosmetic surgery and plastic surgery techniques
- Material safety and handling
- Patient consultation and assessment
Licenses to perform lip filler procedures vary by jurisdiction, but many states require doctors to have a medical degree (MD) or doctor of osteopathic medicine (DO) degree, as well as completion of a certain number of hours of training in cosmetic procedures.
Arrange a Consultation for Dermal Fillers with Dr. Laura Geige Today
For non-medical practitioners, such as estheticians or dermatologists, licenses to perform lip filler procedures may include:
- Licenses to practice esthetics or dermatology
- Certifications in areas such as laser technology or injectable treatments
Accreditation by organizations such as the ASLMS or AACS can also be an indicator of a practitioner’s level of training and expertise in lip filler use.
Regulatory oversight and education play a crucial role in ensuring that medical professionals, including dermatologists and plastic surgeons, follow best practices when administering cosmetic treatments such as lip fillers.
In the context of lip filler injections, regulatory agencies are responsible for establishing standards and guidelines for their safe and effective use. These regulations vary by country, but most countries have laws and guidelines in place to protect patients from potential harm.
For example, in the United States, the Food and Drug Administration (FDA) has approved several lip fillers for cosmetic use, including hyaluronic acid-based products like Restylane and Juvederm. However, these products are only approved for use by licensed medical professionals, and their use must be conducted under the supervision of a qualified practitioner.
The American Society of Plastic Surgeons (ASPS) also plays a significant role in promoting best practices for lip filler administration. The ASPS has established guidelines for the safe use of lip fillers, which include recommendations for proper patient screening, injection technique, and post-procedure care.
Education is also critical to ensuring that medical professionals are aware of the potential risks and benefits associated with lip filler injections. This includes understanding the anatomy of the lips and facial structure, as well as the techniques and materials used in lip filler administration.
A number of organizations offer educational programs and resources for medical professionals who administer lip fillers, including the ASPS and the American Society for Dermatologic Surgery (ASDS). These programs may include online courses, workshops, and conferences.
Some key points to consider regarding regulatory oversight and education in the context of lip filler injections are:
- Regulatory agencies like the FDA establish standards and guidelines for the safe use of approved products like hyaluronic acid-based lip fillers.
- Licenced medical professionals, such as dermatologists and plastic surgeons, must follow established guidelines and best practices when administering lip fillers.
- Education is critical to ensuring that medical professionals are aware of potential risks and benefits associated with lip filler injections.
- A number of organizations offer educational programs and resources for medical professionals who administer lip fillers, including the ASPS and ASDS.
Additionally, patients should be educated about the potential risks and benefits associated with lip filler injections. This includes understanding the possible complications that can arise, such as swelling, bruising, or asymmetry.
A well-educated patient is better equipped to make informed decisions about their own treatment. Patients should also ask questions and seek clarification from their practitioner if they are unsure about any aspect of the procedure.
Overall, regulatory oversight and education are essential for ensuring that lip filler injections are administered safely and effectively. By promoting best practices and providing educational resources, we can help protect patients and ensure the highest quality care possible.
The regulation of cosmetic procedures, including lip fillers, falls under the purview of the Food and Drug Administration (FDA) in the United States.
The FDA’s oversight is primarily focused on ensuring that cosmetics are safe for use by consumers, with an emphasis on preventing adverse events and reducing the risk of long-term health problems.
In the case of lip fillers, which involve injecting dermal fillers such as hyaluronic acid or calcium hydroxylapatite into the lips to enhance their appearance, the FDA has strict guidelines in place to regulate these procedures.
The agency requires that all cosmetic fillers used for lip augmentation be approved by the FDA before they can be marketed and sold to consumers.
Hyaluronic acid dermal fillers, which are one of the most common types of lip fillers used today, were first approved by the FDA in 2003 under a special category of approved products known as “fast-track” or “priority review” status.
This designation was granted due to the substance’s long history of safe use, extensive clinical trials, and low risk of serious side effects.
As a result, hyaluronic acid fillers were cleared for use in lip augmentation procedures, but only after manufacturers provided additional safety data and labeling requirements.
Over time, other types of dermal fillers, such as calcium hydroxylapatite and poly-L-lactic acid (PLLA), have also been approved by the FDA for use in cosmetic procedures.
The FDA’s regulatory framework for cosmetics includes a three-tiered system for evaluating safety and efficacy data:
First-tier: These are products that are generally recognized as safe (GRAS) or have undergone extensive clinical trials to demonstrate their safety and effectiveness.
Second-tier: These products require pre-market clearance, but may still require additional labeling requirements or post-marketing surveillance studies.
Third-tier: These products require a New Drug Application (NDA) or a Biologics License Application (BLA), which involves extensive testing to demonstrate their safety and efficacy before they can be approved for use by consumers.
For lip fillers, the FDA’s regulatory requirements are primarily focused on ensuring that manufacturers provide accurate labeling, follow proper administration procedures, and establish adequate post-marketing surveillance programs to monitor adverse events and side effects.
In addition to its regulatory oversight role, the FDA also provides educational resources for healthcare professionals and consumers to help inform decision-making about cosmetic procedures, including lip fillers.
The agency’s website offers a range of resources, including fact sheets, patient education materials, and guidelines for healthcare professionals, that address topics such as the benefits and risks associated with lip fillers, proper administration techniques, and post-procedure care.
Furthermore, the FDA has issued warnings and alerts to inform consumers about potential risks associated with certain types of dermal fillers, including the use of unapproved or unauthorized products.
This ongoing education and outreach effort helps to promote informed decision-making among consumers and healthcare professionals, ultimately contributing to a safer and more responsible cosmetic industry.
The importance of regulatory oversight and education in the medical field cannot be overstated, particularly when it comes to cosmetic procedures such as lip fillers.
One of the key takeaways from a study published in the Journal of Dermatologic Surgery and Oncology is that educational programs for healthcare professionals have a significant impact on patient outcomes and satisfaction rates.
The study highlighted that healthcare professionals who received specialized training on cosmetic procedures, including lip fillers, were more likely to provide safe and effective treatments.
But what exactly constitutes “specialized training” in the context of regulatory oversight and education?
A number of factors come into play when determining the adequacy of a healthcare professional’s education on a particular topic:
- The quality and relevance of the training program: This includes the curriculum, instructor qualifications, and any hands-on experience or simulation-based training.
- The depth and breadth of the coverage: Does the training program cover all aspects of lip filler administration, including contraindications, complications, and long-term effects?
- The frequency and duration of the training: Is the training program comprehensive enough to ensure that healthcare professionals stay up-to-date with the latest developments in the field?
- The assessment and evaluation methods used: Are healthcare professionals held accountable for demonstrating their knowledge and skills after completing the training program?
In the context of lip fillers, regulatory oversight and education are crucial for ensuring that healthcare professionals have a thorough understanding of:
Contraindications and precautions, such as allergic reactions or nerve damage.
The different types of fillers available, including their composition, properties, and indications.
The administration techniques and protocols required for safe and effective treatment.
The potential complications and side effects associated with lip filler administration.
Long-term effects, such as scarring or permanent changes to the lip structure.
The importance of informed consent and patient counseling in ensuring that patients make fully-informed decisions about their treatment.
In addition to these factors, regulatory agencies and professional organizations play a critical role in overseeing the education and training of healthcare professionals in the cosmetic field.
Regulatory agencies such as the FDA and state medical boards set standards for education and training in cosmetic procedures, while professional organizations like the American Society for Dermatologic Surgery (ASDS) offer certification programs and continuing education courses to ensure that healthcare professionals stay current with best practices.
Overall, regulatory oversight and education are essential components of ensuring patient safety and satisfaction when it comes to cosmetic procedures like lip fillers.
Regulatory oversight and education play a crucial role in ensuring that beauty treatments, including lip fillers, are performed safely and effectively.
The use of lip fillers has become increasingly popular in recent years, with many individuals seeking to enhance the appearance of their lips.
However, the administration of lip fillers is not without risk, and there have been instances where complications such as infection, scarring, and uneven distribution of the filler material have occurred.
To mitigate these risks, regulatory bodies have established guidelines and standards for the use of lip fillers.
In many countries, including the United States, the Food and Drug Administration (FDA) regulates the use of cosmetic products, including lip fillers.
The FDA has approved several lip filler products for use in the treatment of lip augmentation, but these products are only permitted to be used by trained medical professionals.
Additionally, the American Society for Dermatologic Surgery (ASDS) and other professional organizations have established guidelines for the safe use of lip fillers.
These guidelines emphasize the importance of thorough patient evaluation, proper training and experience on the part of the practitioner, and adherence to strict safety protocols.
The education and training of practitioners is also a critical component of regulatory oversight and education.
Many medical schools and dermatology residency programs now offer courses and training programs in cosmetic injectables, including lip fillers.
This specialized training enables practitioners to stay up-to-date with the latest techniques and best practices in the use of lip fillers.
Furthermore, regulatory bodies often conduct audits and inspections to ensure compliance with established standards and guidelines.
In cases where a practitioner is found to be non-compliant, disciplinary action may be taken, including revocation of their license or certification.
This serves as a deterrent to practitioners who may otherwise cut corners in order to increase their profits.
The role of regulatory oversight and education extends beyond the individual practitioner to also include patient education.
Patients have the right to make informed decisions about their own bodies, and they must be provided with accurate information about the risks and benefits associated with lip fillers.
This includes information about the potential for complications, as well as the availability of alternative treatments or options.
Regulatory agencies often provide guidance to practitioners on how to effectively educate patients, including the use of clear and concise language, visual aids, and informed consent forms.
The use of lip fillers is subject to age restrictions in some countries, with minimum age requirements ranging from 18 to 21 years old.
In some jurisdictions, minors under a certain age may require parental or guardian consent before undergoing lip filler treatment.
It’s worth noting that the age restriction for lip fillers can vary depending on the specific product and the country in which it is being used.
The FDA has approved several products specifically for use on younger patients, including children under the age of 18.
However, the decision to use a particular product on a minor should be made in consultation with a qualified practitioner who can assess the individual’s suitability for treatment.
In conclusion, regulatory oversight and education are essential components in ensuring the safe and effective administration of lip fillers.
These efforts help to protect both patients and practitioners from potential risks associated with this popular beauty treatment.
Read more about Making Memories London here. Read more about Critic Forever here. Read more about Madison Art Therapy here. Read more about Create Cocktails at Home here. Read more about Mind Plus Motion here. Read more about My Better Love here.
- Wrapped Cradle Sex Position - June 29, 2025
- When Can I Wear Lip Gloss After Filler - June 28, 2025
- Dermal Fillers Near Norbiton, Surrey - June 28, 2025
